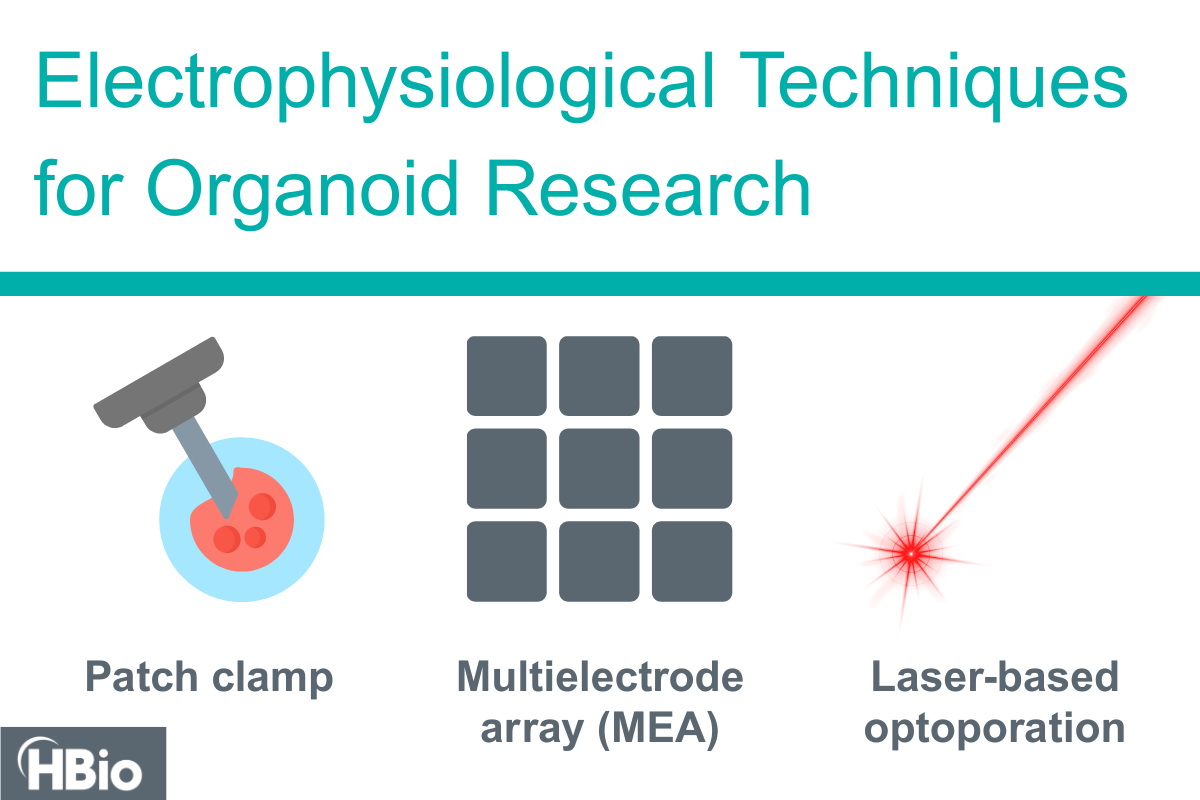
Deep Dive: Electrophysiological Techniques for Organoid Research
Organoid research is revolutionizing our understanding of human biology by providing three-dimensional, physiologically relevant models of human tissues. Key techniques include patch clamp electrophysiology, microelectrode arrays (MEAs), and optical manipulation, each of which unique advantages for studying the functional properties of organoids.
Organoid research is revolutionizing our understanding of human biology by providing three-dimensional, physiologically relevant models of human tissues. To fully harness their potential, researchers employ a variety of electrophysiological techniques to investigate the electrical activity of these miniaturized organ-like structures. Key techniques include patch clamp electrophysiology, microelectrode arrays (MEAs), and optical manipulation—each offering unique advantages for studying the functional properties of organoids.
Patch Clamp Electrophysiology: High-Resolution Single-Cell Analysis
Patch clamp electrophysiology is a gold-standard technique for recording ionic currents in individual cells, making it particularly useful for investigating the electrical behavior of neurons and cardiomyocytes in organoid models. This method involves the use of a glass micropipette to create a high-resistance seal on a single cell’s membrane, enabling precise measurements of ion channel activity, action potentials, and synaptic currents.
The new HEKA EPC 10 USB 3.0 Patch Clamp Amplifier provides the lowest noise levels for whole-cell patch clamp, 2 MHz sampling, automation of experimental procedures, and synchronized fluorescence, among many other features.

Advantages:
- High temporal and spatial resolution
- Ability to isolate and study specific ion channel activities
- Suitable for detailed mechanistic studies of electrical signaling in organoid-derived neurons, cardiomyocytes, or other electrically active cells
Limitations:
- Technically challenging and time-intensive
- Low throughput, making large-scale studies difficult
Learn more about patch clamp solutions from HEKA.
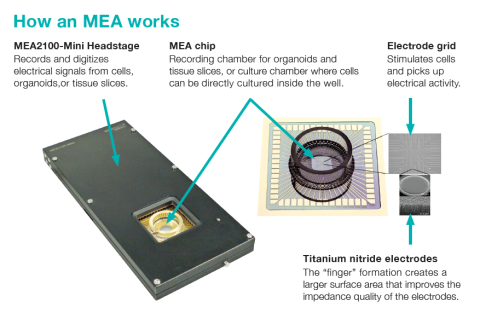
Microelectrode Arrays (MEAs): Network-Level Electrophysiology
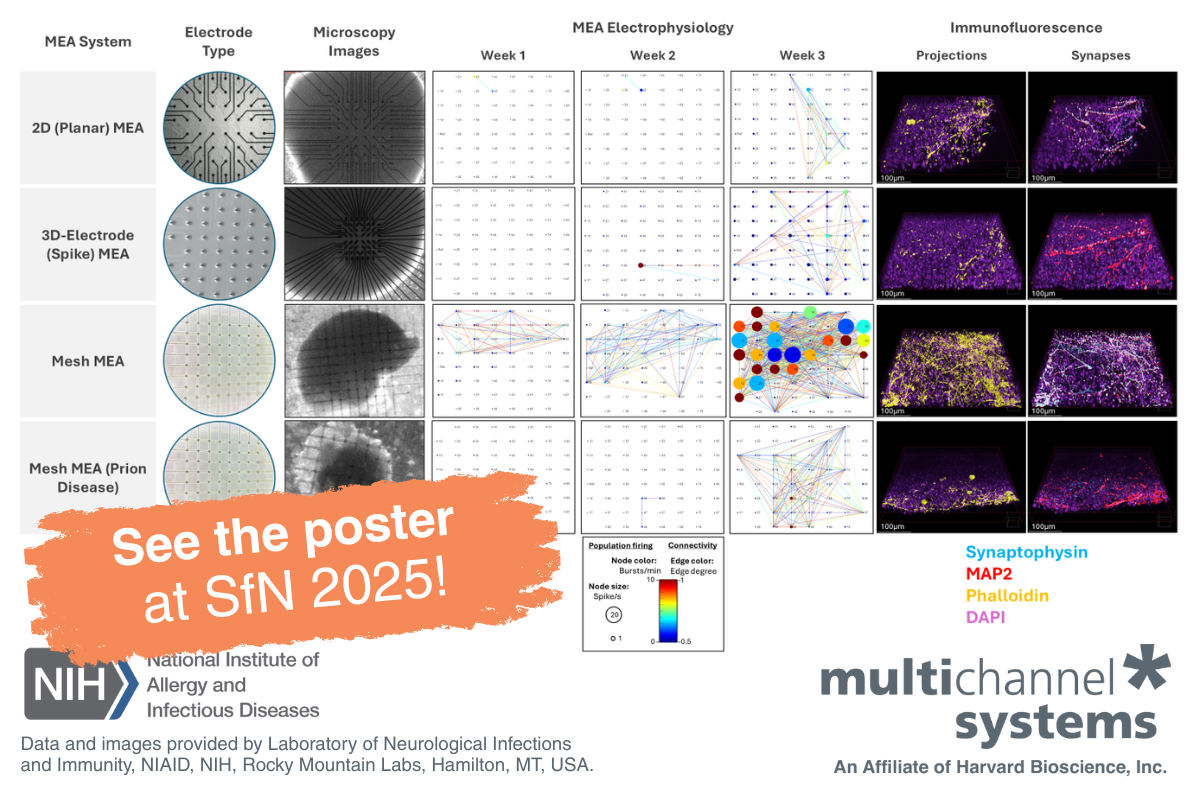
Microelectrode arrays (also called multielectrode arrays or MEAs) offer a non-invasive and scalable approach to measuring extracellular field potentials and action potentials from multiple cells simultaneously. This technique is particularly well-suited for studying neural and cardiac organoids, as it allows for long-term functional monitoring of network activity. Learn more about what is an MEA.
While using an MEA system to measure data from organoids can serve as a powerful in vitro model for drug screening, conventional 2D MEA chips fall short when it comes to capturing electrical activity from within these structures. Due to gravitational effects, organoids will experience morphological changes that can impact their physiology, “flattening” on the surface of the MEA and making it difficult to obtain accurate recordings. 3D MEA solutions with protruding electrodes work well for slice recordings but can still disrupt the morphology of intact organoids.
Mesh MEA, a new innovation from Multi Channel Systems, is specifically designed to preserve the natural shape of organoids while enabling the collection of signals from within their core. Unlike traditional 2D or 3D MEAs, this technology ensures stable, high-quality recordings without structural distortion. Cells grow naturally around the electrodes, allowing seamless data acquisition from internal cells without compromising the organoid’s integrity.
Advantages:
- High-resolution recording of multiple sites within an organoid
- Enables chronic recordings to study developmental and disease processes over time
- Non-invasive, preserving tissue integrity
- Mesh MEA can generate true-to-life long-term recordings from inside an organoid without damaging its structure
Limitations:
- Lower spatial resolution compared to patch clamp
- Primarily measures extracellular field potentials, lacking direct access to single-cell ionic currents
Learn more about MEA solutions from Multi Channel Systems.

Optogenetics: Precise Stimulation of Organoids
Optogenetics combines genetic engineering with light-based stimulation to control cellular activity with high precision. By introducing light-sensitive ion channels such as channelrhodopsins into specific cell types, researchers can use pulses of light to selectively activate or inhibit neurons within organoids. Optogenetics can be combined with MEA techniques to precisely activate or inhibit select populations of neurons in an organoid, and activity throughout the organoid can be measured using a Mesh MEA or with other MEA designs. The combination of these techniques can allow researchers to investigate the contribution of a specific cell type to the electrical activity of an organoid.
Laser optoporation is another technique that can be used in combination with MEA technology to study cardiac action potentials on MEAs. With this technique, a laser is used to optoporate cardiomyocytes that are located on top of MEA electrodes. This briefly opens the cell membrane and allows action potentials to be recorded with MEAs. The IntraCell by Foresee Biosystems can be used in tandem with a Multi Channel Systems MEA2100-Mini system to study action potentials and field potentials in cardiac organoids.
Advantages:
- High specificity in targeting defined neuronal populations
- Enables real-time modulation of neuronal circuits
- Compatible with other electrophysiological techniques like MEAs to assess network responses
Limitations:
- Requires genetic modification, which can be complex
- Light penetration can be limited in larger organoids
Learn more about laser-based optoporation with IntraCell.
Final Thoughts
Electrophysiological techniques are indispensable tools for advancing organoid research, providing critical insights into how these models replicate human physiological and disease states. Patch-clamp techniques enable detailed single-cell analyses, MEAs facilitate large-scale network recordings, and optogenetics provides precise control over cellular activity. As these technologies continue to evolve, they will further enhance the utility of organoids in drug discovery, disease modeling, and regenerative medicine.
By integrating these methods, researchers can unlock deeper understanding of organoid functionality, ultimately bridging the gap between in vitro models and in vivo human physiology. Contact us to learn more about integrating electrophysiological techniques into your research.